Being a Southern California native, it has always seemed ironic that California can be next to such a large body of liquid yet still lack access to reliable drinking water.

Tastes a little salty
When I ask students why water is important during our Water Wonders class, they always understand right away. We need it to drink, to grow our food, and of course to use the restroom. However, it’s tougher to understand where our water supply comes from or what could happen if we can’t get as much as we need. Desalination, or the process of removing dissolved salt from saline water, is a complicated process but it does have the potential to solve many of California’s water supply problems.
How does Desalination Work?
The most common type of desalination works by reverse osmosis (RO) and occurs in four stages. The first stage is pre-treatment and involves filtering out solids from the saline water and adjusting the pH in order to protect and prolong the lifespan of the equipment. Stage two, the pressurization stage, is the step that increases the water pressure for stage three, which actually gets the salt out of the seawater. 1
Osmosis, stage three, is a natural process where solvents, or liquids with dissolved substances, move from areas of low concentration to areas with high concentration in order to find an equal balance. 2 This normally occurs across a semi-permeable membrane that lets some things pass through it, like water, but won’t let other compounds through, like salt.
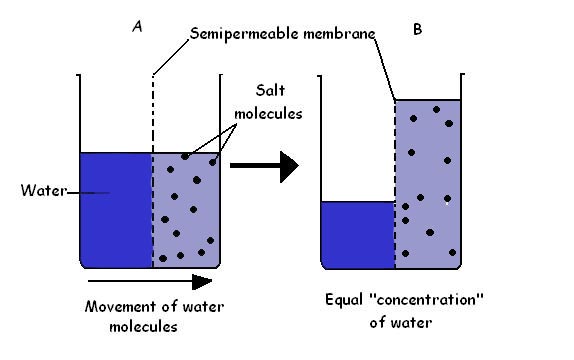 In reverse osmosis, the idea is to create enough pressure to force water through the semi-permeable membrane, against the concentration gradient, effectively leaving the dissolved salt behind . This process will create two byproducts: the first being the desired freshwater and the second being the highly concentrated salt water, or brine. In the final stage of desalination, the pH of the freshwater is adjusted while the brine is disposed of as waste. Drink up!
In reverse osmosis, the idea is to create enough pressure to force water through the semi-permeable membrane, against the concentration gradient, effectively leaving the dissolved salt behind . This process will create two byproducts: the first being the desired freshwater and the second being the highly concentrated salt water, or brine. In the final stage of desalination, the pH of the freshwater is adjusted while the brine is disposed of as waste. Drink up!
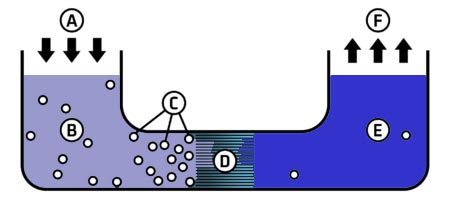
A reverse osmosis system
A – Applied pressure, B – Seawater in, C – Particles, D – Membrane, E – Clean water out, F – Distribution
Right Here, Right Now
Starting in June 2014, the City of Santa Barbara has begun taking baby steps to use the Charles E. Meyer Desalination Facility to make desalination in Southern California, the first big-scale plant on the west coast, a real possibility by summer 2016. 3 4
However, the facility has been closed for 20 years and needs some work to become operational once again. In April, after what is hopefully a wet winter where the need of such a plant can be assessed, the city council will receive bids from different companies to build the facility. The total cost of the project is expected to be $60 million and will raise the average single family home water bill by $14-$20 per month.
Is Desalination really the Solution?
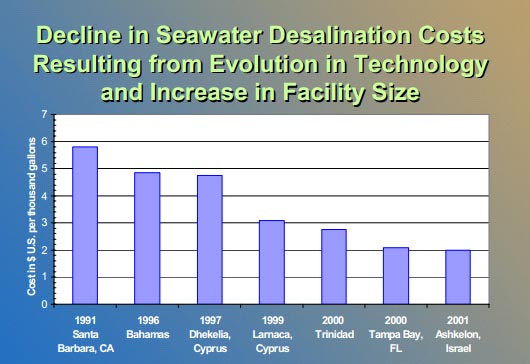
When desalination was first discovered, some of the first criticisms came from the large energy demands the process required and the high cost of equipment that needed to be replaced frequently. In other words, the energy and equipment costs were extremely expensive for the amount of water that was produced. With advances in technology, the semi-permeable membranes have gotten more effective, last longer, and the cost has gone down considerably. 5
In Santa Barbara, environmentalists have taken a special interest in ways to make the desalination plant as environmentally friendly as possible. Local group Channelkeeper is lobbying to have the water in-take be sub-surface (below the surface of the sand) in order to avoid negatively impacting marine life with an open ocean intake 6. The cost of making this ocean-friendly design would be additional time and expenses in constructing the facility.
 Another potential impact from this facility would be the carbon emissions from the high energy demands of desalination. Heal the Ocean, another environmental group, has been in contact with the city council about making the desalination plant powered with renewable energy instead of traditional fossil fuels in order to decrease the large carbon emissions needed for such a project.
Another potential impact from this facility would be the carbon emissions from the high energy demands of desalination. Heal the Ocean, another environmental group, has been in contact with the city council about making the desalination plant powered with renewable energy instead of traditional fossil fuels in order to decrease the large carbon emissions needed for such a project.
Until the city council awards a contract and the final design is revealed, only time and a continued drought will determine the need and benefits of such a project.
California’s Future
So with the possibility of desalination in Santa Barbara, what does this mean for the rest of Southern California? If the Charles E. Meyer Desalination Plant successfully produces 5,000 acre-feet per year, that means the desalination plant could produce over 1.6 billion gallons of water in a year. Because the average Santa Barbara resident used 167 gallons of water per day in 2010 7, the Santa Barbara government predicts that the desalination plant could meet a little over 20% of the city’s water supply needs. 8
Now What?
 Even though a project this large cannot produce enough water to provide water for the surrounding areas, it could help reduce the pressure of other local water resources. In the meantime, everyone should still do their part in conserving water use as much as possible.
Even though a project this large cannot produce enough water to provide water for the surrounding areas, it could help reduce the pressure of other local water resources. In the meantime, everyone should still do their part in conserving water use as much as possible.
Talking about the energy and cost to make clean water out of the ocean helps to reinforce the basic conservation ethics about water that we teach here at High Trails.
At High Trails Outdoor Science School, we literally force our instructors to write about elementary outdoor education, teaching outside, learning outside, our dirty classroom (the forest…gosh), environmental science, outdoor science, and all other tree hugging student and kid loving things that keep us engaged, passionate, driven, loving our job, digging our life, and spreading the word to anyone whose attention we can hold for long enough to actually make it through reading this entire sentence. Whew…. www.dirtyclassroom.com
- http://www.oas.org/usde/publications/Unit/oea59e/ch20.htm ↩
- http://scienceofwater.com/chemistry-behind-reverse-osmosis-water-purification/ ↩
- http://www.oas.org/usde/publications/Unit/oea59e/ch20.htm ↩
- http://scienceofwater.com/chemistry-behind-reverse-osmosis-water-purification/ ↩
- http://wrri.nmsu.edu/publish/watcon/proc49/leitz.pdf ↩
- http://www.independent.com/news/2014/sep/25/council-clears-path-desalination/ ↩
- http://www.missionandstate.org/ms/wp-content/uploads/2013/05/September-2013-Santa-Barbara-County-Water-Supply-and-Demand-Current-Uses-and-Future-Estimates-Mission-and-State.pdf ↩
- http://kanat.jsc.vsc.edu/student/glowact/body.h1.jpg ↩

Comments are closed.